Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
1.
|
Chemistry is
a. | a characteristic of a substance that can be observed without changing the substance
into another substance. | b. | the study of matter and how matter
changes. | c. | anything that has mass and takes up space. | d. | a rapid reaction
between oxygen and a substance called a fuel. |
|
|
|
2.
|
What happens when chemical bonds break and new bonds form?
a. | a physical change | b. | a chemical reaction | c. | matter is
destroyed | d. | surface area increases |
|
|
|
3.
|
The only sure evidence for a chemical reaction is
a. | the formation of a gas. | b. | a color change. | c. | the production of
one or more new substances. | d. | changes in
properties. |
|
|
|
4.
|
A chemical reaction that absorbs energy in the form of heat is described
as
a. | endothermic. | b. | exothermic. | c. | combustion. | d. | unbalanced. |
|
|
|
5.
|
Every chemical reaction involves a change in
a. | mass. | b. | energy. | c. | concentration. | d. | state. |
|
|
|
6.
|
CaCO3 represents a chemical
a. | symbol. | b. | formula. | c. | subscript. | d. | reaction. |
|
|
|
7.
|
A shorter, easier way to show chemical reactions, using symbols instead of
words, is called a
a. | chemical equation. | b. | chemical formula. | c. | symbol. | d. | subscript. |
|
|
|
8.
|
The substances listed on the left side of a chemical equation are the
a. | products. | b. | coefficients. | c. | precipitates. | d. | reactants. |
|
|
|
9.
|
Which of the following is NOT a sign of a chemical reaction?
a. | color change | d. | solid formation(precipitate) | b. | gas
formation | e. | energy
change(gets cooler or warmer) | c. | change of state |
|
|
|
10.
|
During a chemical reaction bonds between the atoms in the reactants
a. | break | c. | don’t change | b. | form |
|
|
|
11.
|
During a chemical reaction, atoms of the products
a. | have rearranged themseives to form new bonds or new compounds. | c. | are less numerous
than the atoms of th reactants. | b. | are more numerous than atoms of the
reactants. |
|
|
|
12.
|
A covalent bond between atoms is when they
a. | take and give electrons. | c. | lose electrons | b. | share
electrons. |
|
|
|
13.
|
When atoms form ionic bonds
a. | one atom takes and one atom gives up electrons. | c. | both A and B | b. | the atoms that form
bonds are oppositly charged. |
|
|
|
14.
|
The force that holds atoms together in ionic bonds is
a. | gravity | c. | magnetism | b. | electrostatic |
|
|
|
15.
|
In the formula MgCl2, the Mg stands for
a. | Chlorine | c. | Magnesium | b. | Mercury |
|
|
|
16.
|
In the compound C6H12O6 the subscript
6 after the C means
a. | there are 6 atoms of carbon in the compound | c. | there are 12 atoms of hydrogen in
the compound | b. | there are 6 atoms of oxygen in the compound |
|
|
|
17.
|
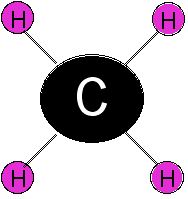 The chemical formula for this molecule is
|
|
|
18.
|
Dihydrogen monoxide is
a. | water | c. | both A and B | b. | H2O |
|
|
|
19.
|
The Magnesium(Mg) ion has a charge of +2. The Chlorine (Cl )ion has a charge of
-1. the formula for magnesium chloride is
|
|
|
20.
|
The formula for sulfur trioxide is
|
|
|
21.
|
Which is a balanced chemical equation?
a. | H +O --> H2O | c. | 2H +O -->
H2O | b. | 2H +O --> 2H2O |
|
|
|
22.
|
The numbe 2 in front of the H in this chemical equation (2H +O -->
H2O )is
a. | the coefficient | c. | the superscript | b. | the subscript |
|
|
|
23.
|
The use of symbols, numbers, and arrows to represent a chemical reaction
is
a. | a physical change | c. | a chemical equation | b. | a chemical
change |
|
|
|
24.
|
During a chemical reaction, the mass of the products will always
a. | be more than the mass of the reactants. | c. | equal he mass of the
reactants | b. | be less than the mass of the reactants. |
|
|
|
25.
|
When you mix polyvynyl alcohol with sodium tetra borate you get slime.
a. | polyvynyl alcohol and sodium tetraborate are the reactants. | c. | both A and
B | b. | slime is the product. |
|